what is the highest amount of dextrose to administer through a picc line.
US Pharm. 2006;7:HS-x-HS-20. Parenteral
• Peripheral parenteral nutrition (PPN): The commitment of nutrients into a small vein using a feeding catheter. • Fundamental parenteral nutrition (CPN): Used when the catheter tip is placed in a big, high-flow vessel such as the superior vena cava.
• Total parenteral nutrition (TPN): A misleading term because many patients who currently receive diet by vein too concomitantly receive diet by mouth or by enteral (tube) feedings.
• Hyperalimentation: While this term is still used, it implies overfeeding calories beyond a patient's requirements--a practice that has been largely replaced by more conservative feeding.
Indications
PN is usually used in such conditions equally severe pancreatitis, brusque-bowel syndrome, inflammatory bowel disease exacerbations, and gastrointestinal (GI) fistulae, too as in critically ill patients, infants with very low birth weight, and patients with cancer receiving hematopoietic cell transplantation.2 While enteral nutrition (EN) may be more beneficial in some weather (most notably, severe pancreatitis and disquisitional illness), PN is still normally used.
When to initiate PN or EN (collectively known every bit specialized diet support[SNS]) is controversial and can dramatically impact the number of patients receiving SNS. 2 The hospital pharmacist should be aware that administration of PN is never a medical emergency.two Although in that location is prove that administration of EN within a few hours of severe injuries (e.thousand., trauma, burns) may ameliorate patient outcomes, no such evidence exists for PN. Both PN and EN should be delayed until patients are hemodynamically stable (i.eastward., do not crave high or widely fluctuating dosages of vasopressor medications). 2
An institutional usage pattern, in which many patients receive PN for a week or less and then transition to adequate oral intake, should prompt the hospital pharmacist to investigate whether prescribers are appropriately selecting patients for this expensive, potentially dangerous therapy (come across "Complications" for the dangers of PN). Few data support improved outcomes in patients receiving brusk-duration PN.2 Still, patients receiving no nutrition for 10 to 14 days are likely to have poorer clinical outcomes. Current guidelines from the American Guild for Parenteral and Enteral Nutrition state that SNS, with a preference for EN, should be initiated when oral intake has been or is expected to exist inadequate for 7 to 14 days.2 A patient's preexisting nutritional status should be taken into account, with SNS typically started earlier in previously malnourished patients.
Access Devices
For curt-term CPN in the infirmary, a temporary primal venous catheter is placed percutaneously into the subclavian vein by a medico at the bedside, with the catheter tip at the superior vena cava adjacent to the right atrium.three If PN duration is expected to be more than than a few weeks, a subcutaneously tunneled catheter is placed with the tip at the superior vena cava; this procedure is usually performed in the operative suite. With more permanent devices, such as the Hickman catheter or Port-a-Cath, the injection port may be external or completely beneath the pare, respectively. A peripherally inserted central catheter (PICC) is another fundamental venous access device that tin exist placed by specially trained nurses at the bedside.4 The PICC is a central line through which hypertonic fluids can be administered. The device is ordinarily inserted into the basilic vein on the within of the elbow and threaded so that the tip of the catheter rests at the superior vena cava.
Peripheral access for PPN is uncommon in the U.s.a., compared to other parts of the world.5 When PPN is used in the U.S., osmolality of the infusate is usually limited to approximately 900 mOsm/L, and elapsing of therapy is limited to about seven to x days. A midline catheter (i.e., a catheter placed via the basilic vein with the tip in a vein in the upper arm) is a peripheral access device through which fluids with osmolality in a higher place 900 mOsm/50 should non exist administered, due to risk of phlebitis.
Components of PN
Components of PN tin exist divided into macronutrients (i.e., protein, carbohydrate, fatty) and micronutrients (i.due east., electrolytes, vitamins, trace minerals). A patient's fluid load must as well be considered when PN is administered.
Poly peptide is provided as crystalline amino acrid solutions. Manufacturers supply standard 4 amino acid products that contain a mixture of essential amino acids (EAA) and nonessential amino acids (NEAA), which are appropriate for most adult patients receiving PN. These manufacturers too provide amino acid formulations that are specially designed for young children (TABLE 1). Although the amounts of EAA and NEAA in standard products vary slightly between manufacturers, the differences are generally not clinically meaning. However, clinically significant differences may exist in the endogenous electrolyte content of various products, about notably in the phosphorus, acetate, and chloride content. When switching products due to shortages or contract changes, a brief report of electrolyte differences is prudent.
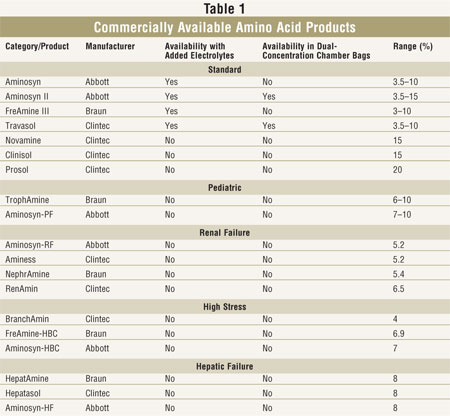
Amino acrid products are supplied in concentrations from iii.5% to 20%; more than concentrated solutions are useful in compounding for fluid-restricted patients. Amino acrid formulations are available with or without added electrolytes. Added electrolyte solutions may be useful in institutions where PN use is minimal, equally they minimize the number of admixtures necessary. Notwithstanding, fixed electrolyte content may not be appropriate for many patients, especially those who are critically ill. Products without added electrolytes yet contain some electrolytes. Amino acrid solutions provide 4 kcal/gram of amino acid.
Pediatric formulations are commonly used in very immature children. Specialty products designed for patients with renal failure, hepatic failure, and high stress are not widely used because they have trivial proven clinical benefit. Most experienced diet support clinicians adopt to use less expensive standard formulations in these populations.
Dextrose is the most mutual carbohydrate used in PN solutions. Dextrose solutions normally used for compounding range from 10% (for PPN solutions) to lxx%, with last concentrations of dextrose commonly in the range of five% (for PPN) to 30%. Dextrose for 4 apply provides 3.4 kcal/gram. Manufacturers cannot supply dextrose and amino acid premixed because these products react when estrus sterilized. ProcalAmine combines glycerol three% with amino acid 3%, a mixture that can be heat sterilized and supplied commercially. This production is used as PPN in some institutions. If used every bit PPN, IV lipid should generally be piggybacked to increase calories. Caloric density of glycerol is iv.3 kcal/gram. Although glycerol may be useful in controlling claret glucose, especially in patients with diabetes, the low concentrations of glycerol and amino acrid in ProcalAmine limit its usefulness.
Another method used by manufacturers to facilitate the mixture of dextrose and amino acid solutions is provision in dual-bedroom numberless. To combine dextrose and amino acids, a septum between two chambers is broken and contents are mixed. In that location is room to add together fat emulsion if desired. Amino acid solutions available in dual-chambers are noted in Tabular array 1. These products are supplied with and without added electrolytes.
Lipid is supplied in the U.S. under the trade names Intralipid, Liposyn Two, and Liposyn 3. These soybean oil or safflower plus soybean oil–based emulsions primarily contain the long-chain fatty acids linoleic and linolenic acid. These products incorporate egg yolk phospholipids as emulsifiers and glycerol for tonicity. Iv lipid provides 1.i kcal/mL for 10% emulsion, 2.0 kcal/mL for 20% emulsion, and 2.9 kcal/mL for 30% emulsion. Due to concerns that long-chain triglyceride emulsions used in the U.S. may be immunosuppressive, there is interest in alternative emulsions.6 Alternatives containing medium-concatenation triglycerides and olive oil are available in Europe and may accept immunologic and metabolic advantages.
Micronutrient components of PN solutions include electrolytes, vitamins, and trace minerals. The electrolytes usually present include sodium, potassium, magnesium, calcium, phosphorus, chloride, and acetate. Typical daily adult micronutrient requirements are listed in TABLE 2.2,7-nine Requirements for predominantly intracellular electrolytes (potassium, magnesium, and phosphorus) are somewhat driven by carbohydrate content of the PN, with requirements increasing as sugar increases. Since these electrolytes are primarily excreted past the kidneys, infused amounts required may be lower in patients with renal insufficiency. Monitoring for serum electrolytes is useful for guiding the amount of electrolyte placed in PN. It is noteworthy that serum sodium is often non reflective of total body sodium stores, although series values can exist useful for monitoring fluid status. Patients with metabolic alkalosis may benefit from increasing chloride and decreasing acetate in the PN, whereas patients with metabolic acidosis may benefit from the opposite profile of these electrolytes. Sodium bicarbonate should not be added to PN solutions every bit an alkalinizing agent because it tin can interact with calcium to course insoluble calcium carbonate; sodium acetate or potassium acetate should be used instead. nine
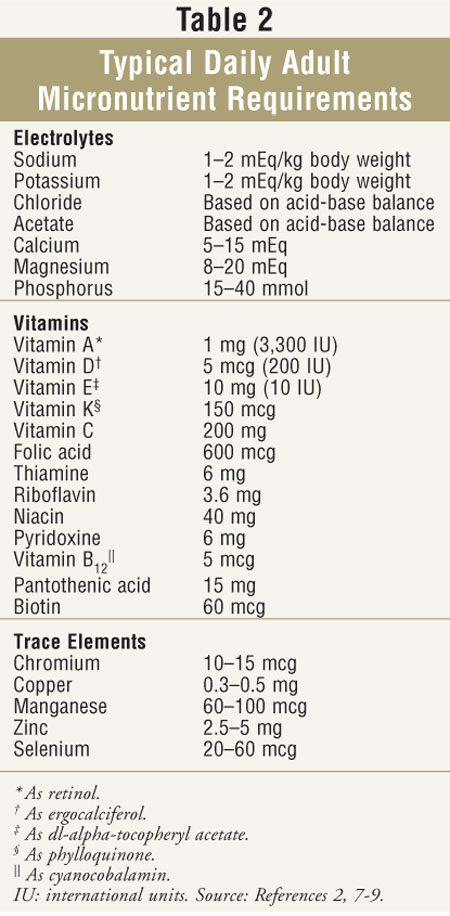
Vitamins are usually added using parenteral multivitamin preparations, which contain 12 or thirteen essential vitamins. The number of vitamins in most commercial preparations has recently been reformulated based on FDA guidelines.10 The most notable change has been the addition of vitamin K to much of the adult parenteral multivitamin marketplace. The 150 mcg corporeality of phylloquinone in a daily supply is relatively niggling and should non clinically touch warfarin anticoagulation when administered consistently. Nonetheless, the international normalized ratio should be monitored closely in patients receiving warfarin in whom PN is beingness started or discontinued. Shortages of parenteral multivitamins take occurred in contempo years; in such instances, the add-on of individual vitamin ingredients such equally thiamine and folic acid may exist important to avoid complications.
Zinc, chromium, manganese, and copper are the 4 trace elements almost commonly added to PN solutions. Selenium is likewise added, although not every bit universally for brusque-term PN patients. Commercially available products containing a combination of trace elements are frequently used. Some institutions add zinc in quantities beyond those establish in commercial mixtures for certain surgical patients. Copper and manganese undergo biliary excretion and can accumulate in patients with severe hepatic affliction; they should be omitted in patients with significantly elevated total bilirubin.2
Iodine and molybdenum are trace elements added less frequently, unremarkably in long-term PN. Aluminum is a contaminant of parenteral additives that can add upward to potentially unsafe amounts in neonates and in patients with renal failure. This has prompted the FDA to require disclosure of aluminum content of many of the parenteral products used in compounding PN.xi Monitoring for fe deficiency is of import in long-term PN patients. Although fe is not routinely added to PN, the mineral may be added to PN solutions containing dextrose and amino acids, just non to solutions containing lipid emulsion due to stability issues. Iron dextran is the course of iron most usually added to PN.
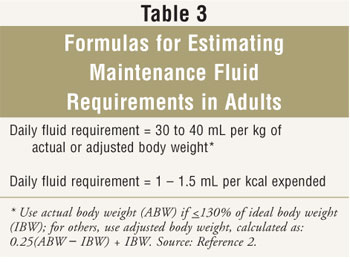
Fluid requirements for patients receiving PN should be monitored. Daily weights are useful in hospitalized patients; weight change of more than 0.5 kg in a day is due largely to fluid gain or loss, rather than change in lean trunk mass or fat. Inputs and outputs should be monitored in astute care to gauge fluid status. Serial monitoring of blood for albumin, sodium, and hematocrit may also be helpful in determining fluid status when used in combination with body weight and inputs and outputs; these values can reflect dilution and concentration.
Formulas for estimating maintenance fluid requirements in patients without unusual losses are found in Tabular array 3.
Compatibility and Stability Issues
Calcium and phosphate solubility is a major result concerning the compatibility of PN formulations. Solubility is influenced by several factors such as temperature; calcium phosphate solubility decreases with increasing temperature.12 Formulations that appear stable when refrigerated could form precipitates at room temperature. Another important factor is pH; calcium phosphate solubility increases as pH decreases. Higher final amino acid and dextrose concentrations are associated with lower pH and thus college calcium phosphate solubility.
Calcium gluconate is preferred in PN solutions due to superior solubility compared to calcium chloride. The order in which calcium and phosphate are added is important; phosphate is more often than not added first, while calcium is added near the terminate of the compounding sequence. The amounts of calcium and phosphate added must exist considered, with a greater chance of precipitation if the amount of one or both is increased above standard. If lipid is admixed with the PN to course a total nutrient admixture (TNA), visual detection of calcium phosphate precipitates becomes more difficult. The pharmacist must follow the manufacturer's calcium and phosphate guidelines for specific products and concentrations comprising whatever PN admixture. Simplified formulas for estimating the maximum amount of calcium and phosphate that tin exist placed in PN formulas are fraught with error. In-line, 0.22-micron (preferred), or one.2-micron filters should be used when infusing PN solutions containing dextrose plus amino acid.9 TNA should be infused through a 1.2-micron filter.9
TNA poses greater challenges in terms of stability due to the lipid component, as compared to dextrose plus amino acid solutions. Chemical stability tin can be compromised by excessive cations, specially divalent cations, resulting in "creaming" or "cracking" of the TNA. With creaming, lipid can be redispersed with gentle inversion and administered to a patient.9 Notwithstanding, with a cracked TNA, separated lipid does not redisperse with gentle inversion and must not be administered. 9 For maximal stability, TNA should comprise concluding concentrations of macronutrients inside the post-obit ranges: dextrose, 3.three% to 35%; amino acid, one.75% to 5%; and lipid, 2% to half dozen.seven%.8
Pharmacists should also consider the expiration time for Four lipids hung separately from the dextrose and amino acid. A TNA is generally considered microbiologically safe for 24 hours after initial hanging. However, lipid emulsion alone is a better growth medium due to its nearly physiologic osmolality and pH. This is in contrast with a TNA that is hypertonic and has a lower pH. The current CDC recommendation is that a lipid emulsion hung lonely should non infuse for more than 12 hours after spiking the container.xiii Literature back up for this recommendation has been summarized elsewhere.14
Nutritional Cess
Assessing the quantitative needs of patients receiving PN is of import. Overfeeding macronutrients or micronutrients can lead to complications, while underfeeding tin be associated with malnutrition or micronutrient deficiency. Cess of nutritional condition has historically been performed based on a combination of physical examination characteristics, biochemical parameters, and immunological markers. Immunological markers include total lymphocyte counts and anergy screening. Unfortunately, these markers are nonspecific and take largely been abandoned equally nutritional markers.
Widely used biochemical markers include serum albumin and other circulating proteins. Albumin concentrations fluctuate based on hydration status and tin driblet precipitously following stress or injury equally protein redistributes. The long half-life of albumin (nearly 21 days) does not make it optimal for serial monitoring in hospitalized patients, although information technology is oft a good marker of long-term nutritional status. Therefore, shorter half-life proteins are frequently used for tracking nutritional response to feeding. Prealbumin is perhaps well-nigh commonly used (one-half-life is about two days). In critically ill patients, prealbumin concentrations are sometimes used with C-reactive protein (CRP) concentrations. CRP is an acute phase reactant and marker of inflammation. Synthesis of prealbumin is not a priority of a stressed patient's body until inflammation begins to reject. Therefore, a significant ascent in prealbumin is not expected--even with acceptable nutritional support--until CRP declines. Prealbumin can be afflicted by conditions other than malnutrition, such equally renal and hepatic disease.
Early in the PN era, measurements such as mid-arm muscle circumference and peel folds of the triceps were widely used to help determine nutritional condition. These methods are now rarely used in the clinical setting. More commonly used is the subjective global assessment technique, which considers recent changes in weight and dietary intake, presence of GI symptoms, functional capacity, and concomitant diseases.xv
Indirect calorimetry (IC) is the golden standard clinical tool for determining calorie requirements of SNS patients. IC measures carbon dioxide production and oxygen consumption. Resting free energy expenditure (REE) is calculated from these values. Patients are fitted with a mask or mouthpiece, or a rigid canopy is placed over their caput. Patients receiving mechanical ventilation can have IC performed by hooking into the ventilatory apparatus. Recently, less expensive hand-held IC devices have been marketed, which may be useful for alert patients who can cooperate with measurement, although this is oftentimes not the case in hospitalized patients.
The REE obtained from IC is a guide for determining how many calories to feed. Typically, hospitalized patients are fed near their REE, although sometimes they are fed well below their REE (permissive underfeeding). Permissive underfeeding may be particularly useful in morbidly obese patients; the optimal amount of calories for this population is however being investigated.16 The maximum amount of dextrose recommended in adult PN is 7 g/kg/day, and maximum lipid corporeality is 2.5 g/kg/day.9 Nonetheless, these maximums are rarely approached in current clinical practice. Dextrose is typically supplied at 3 to 5 g/kg/day, while lipid is often limited to less than 1 g/kg/24-hour interval in critically ill and immunocompromised patients.
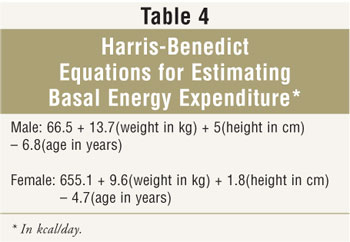
Since many institutions and home care agencies practise not perform IC, caloric requirements must be estimated. Many clinicians use Harris-Benedict equations to gauge basal free energy expenditure (BEE) (Table 4). Activity level and/or stress factors are oft added to calculated BEE, which sometimes results in overfeeding. Other formulas, such as the Swinamer and Frankenfield equations, have been developed for specific populations. Alternatively, many clinicians estimate caloric requirements on a kcal/kg basis; typical ranges provided by this approach are twenty to 30 kcal/kg/day. Determining which weight to use to calculate caloric requirements in obese patients is controversial. Many clinicians utilise an "adjusted body weight," such every bit platonic body weight plus about 25% to 50% of excess weight.17
Providing adequate poly peptide is important when formulating PN. In fluid-restricted patients, information technology is sometimes necessary to choose betwixt goal calories or goal protein. In such a situation, many clinicians would choose to come across goal poly peptide requirements at the expense of goal free energy requirements. Typically, patients receiving PN are given 1 to 2 grand of protein per kg of trunk weight per day. In general, the more highly stressed a patient is, the more protein he or she requires to maintain nitrogen equilibrium (i.e., to forbid lean torso mass loss). In patients weighing less than ideal body weight, actual trunk weight should be used to calculate caloric and protein requirements. In obese patients, adapted body weight is usually used to decide protein requirements.
A nitrogen residuum report tin estimate whether SNS is meeting a patient'due south protein requirements. A 24-hour urine collection is performed and urinary urea nitrogen (UUN) or total urea nitrogen (TUN) is measured by the laboratory. Although TUN is preferable, UUN is more commonly measured because it is easier for the laboratory to perform. The formula for calculating nitrogen balance when UUN (in thou/twenty-four hour period) is reported is:
Nitrogen residue = Poly peptide intake (chiliad) – (UUN + 4)
6.25
The number iv in this formula is an gauge of fecal and cutaneous loss of nitrogen (2 g), plus non-urea urinary nitrogen (2 g). To calculate nitrogen intake, the number of grams of protein supplied to the patient is divided by half-dozen.25. Nitrogen makes up about sixteen% of the total weight of amino acids in commercially available Four products. The goal is to take a positive residual; that is, information technology is preferable that a patient receive more nitrogen than is excreted, which implies a net gain of lean torso mass. However, this is unrealistic for many severely ill patients during the summit of affliction. In such cases, the goal is to minimize the loss of lean body mass (i.e., minimize the negative nitrogen balance every bit much as possible).
Certain patients may require protein in amounts greater or less than ane to 2 1000/kg. Patients with renal insufficiency in whom dialysis has not been initiated may not tolerate protein at 1 g/kg. However, protein in lower amounts is non optimal because acute renal insufficiency is most frequently seen concomitantly with catabolic illnesses. Such patients require dialysis in order to be fairly fed from both a fluid and protein standpoint. Dialysis therapy likewise removes excess nitrogenous waste matter from protein metabolism. Patients receiving some of the newer continuous renal replacement therapies (CRRTs) may benefit from more than 2 g/kg due to large protein losses with CRRT.eighteen Patients with end-phase liver disease may need to have protein restricted to less than 1 g/kg in the presence of hepatic encephalopathy.
Complications
Complications of PN can exist divided into 3 main categories--mechanical, metabolic, and infectious. Mechanical complications include pneumothorax with catheter placement, thrombosis, and phlebitis. A breast x-ray should e'er be performed later on catheter insertion to ensure that the catheter tip is correctly located before PN administration. Thrombosis tin can occur at the catheter tip and generally begins with formation of a fibrin sheath on the exterior of the catheter. Immigration of a catheter occlusion due to a fibrin sheath or thrombosis can be accomplished by infusion of a thrombolytic amanuensis, such as tissue plasminogen activator, through the catheter.19 Some patients with permanent cardinal catheters who receive abode PN are given low-dose warfarin to help prevent thrombosis; efficacy of this technique is debated, and more than evidence supports this exercise in patients with malignancies than in patients receiving dwelling PN. xx,21 The add-on of heparin to PN does non announced to subtract thrombosis run a risk.xx
Thrombophlebitis is a limiting complication of PPN. Phlebitis with PPN tin can be minimized through frequent rotation of catheter sites and careful choice of catheter size and blazon. v,22 A commonly cited recommendation is to limit osmolality of PPN to less than 900 mOsm/L; recommendations for both lower and college limits of osmolality are found in the literature.5,22 It appears that PPN formulated every bit TNA is better tolerated than dextrose/amino acid mixtures with lipid piggybacked into the Four line, regardless of osmolalities. The addition of heparin and hydrocortisone to PPN solutions has non been finer shown to reduce phlebitis.v
Electrolyte abnormalities are metabolic complications of PN. Significant preexisting abnormalities are preferably corrected prior to PN initiation. Hypokalemia, hypomagnesemia, and hypophosphatemia are mutual complications of PN. Adding more of these electrolytes to the PN or equally separate infusions should right these abnormalities. Hyperkalemia, hypermagnesemia, and hyperphosphatemia are virtually commonly seen with renal insufficiency; brake should help correct these abnormalities. Alteration of the acetate-to-chloride ratio may be helpful in correcting metabolic acidosis or metabolic alkalosis that may or may not be related to PN. Specific guidelines for the correction of electrolyte abnormalities in critically sick patients have been published. 23
Vitamin and trace chemical element deficiencies can occur during long-term PN. Some domicile care companies may monitor serum concentrations of sure micronutrients on a regular basis, perchance one time or twice a year.24 Specific patient parameters may prompt the clinician to monitor a sure micronutrient. For example, patients with draining fistulas may exist monitored closely for evolution of zinc deficiency. Business organisation about accumulation of copper and manganese in patients with significant hepatic illness is prudent; in such cases, these trace elements may be omitted, and chromium, zinc, and selenium may be added as separate entities. Generally, monitoring for vitamin and trace chemical element abnormalities becomes more than critical as a patient remains on PN for a longer amount of fourth dimension.
Overhydration and dehydration are concerns in patients receiving PN. The pharmacist is frequently called upon to concentrate or dilute PN to better friction match fluid requirements.
The importance of tight glycemic command, especially in critically ill patients, has recently been emphasized.25 Starting with a low amount of dextrose in the PN (less than ii yard/kg/day) and titrating upwards to goal charge per unit (usually 3 to 5 g/kg depending on caloric requirements) over several days may be helpful in preventing extreme glycemic excursions. Many patients will crave insulin to keep blood glucose inside acceptable limits. Insulin should exist added to PN in the pharmacy grooming area; information technology should not be added subsequently the PN is hung, due to sterility concerns. One recommendation is to start with 0.ane unit of insulin per gram of dextrose in the PN container and increase in increments of 0.05 unit per gram, with subsequent mixes as necessary.26 For patients with more extreme increases in blood glucose, a separate insulin drip is preferred to fine-tune the insulin. Many clinicians now strive to keep blood glucose levels as shut to normal every bit possible in critically ill patients and below about 150 mg/dL in hospitalized patients who are less severely sick. 26
Gross overfeeding tin can lead to excessive carbon dioxide production and could interfere with weaning from mechanical ventilation. Since metabolism of carbohydrate results in production of more carbon dioxide than metabolism of lipid, it was sometimes recommended to give relatively more than lipid and less dextrose in mechanically ventilated patients.27 With lower numbers of full calories currently recommended, this is probably not clinically relevant.
Liver function test abnormalities take been often reported in patients receiving PN. These abnormalities are generally divided into two categories in adult patients--hepatic steatosis and cholestasis.28 Hepatic steatosis, or fat accumulation in the liver, is manifested as an elevation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Hepatic steatosis due to PN is not as common as in the past, due to conservative amounts of nutrients now prescribed. Even so, elevations in ALT and AST--especially in the first 7 to 10 days of PN--should cause the clinician to reassess the formulation to ensure the patient is not being overfed.
Virtually patients on long-term PN develop some cholestasis. In the absenteeism of enteral intake, the gallbladder is not stimulated to empty. Bile becomes thick and sludgy and tin eventually cause biliary obstruction. Elevations in total bilirubin and alkaline phosphatase occurring a few weeks or more after initiation of PN may indicate cholestasis. The all-time prevention and treatment is the utilise of enteral feedings (even small amounts), if possible.
Metabolic os disease is a complication unique to home PN. Many patients receiving long-term PN volition develop osteoporosis or osteomalacia. The definitive cause is unknown, although several preventative strategies such as conscientious attention to the amounts of calcium, magnesium, phosphorus, and vitamin D provided in the PN take been suggested.29 Limitation of protein in the PN to about one one thousand/kg/day in the long-term patient may also help foreclose hypercalciuria, thus preserving bone mass.29
Catheter-related sepsis (CRS) is the most common cause of hospitalization in home PN patients. CRS tin likewise be a complication of patients receiving PN through a temporary access device. With temporary devices, the catheter is typically replaced if infection is suspected. With permanent devices, attempts to salvage the catheter are often made because of difficulty in removing and replacing the device.thirty In these cases, systemic antibiotic therapy is attempted if the patient is non seriously ill. The catheter is removed and replaced merely if infection fails to clear after an adequate trial of antibiotics. Most clinicians would remove the catheter if fungal CRS is confirmed, as this is exceedingly difficult to clear with the catheter in identify.
Monitoring
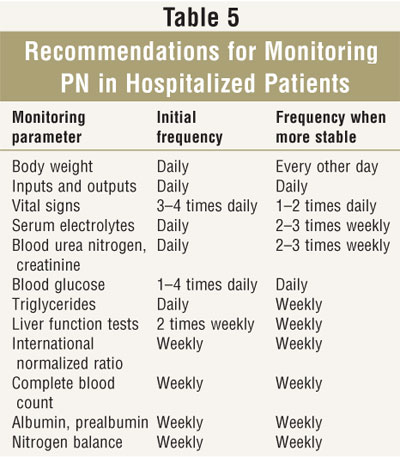
General recommendations for monitoring PN are listed in TABLE 5. Monitoring should be individualized, and baseline values should be obtained for most of these parameters prior to PN initiation. In critically ill patients, monitoring is generally performed more frequently than in stable patients. Laboratory monitoring may exist done quite infrequently in stable patients on habitation PN.
Drug Compatibility with PN
Several drugs have been proven stable when admixed with PN solutions and are commonly added. The virtually mutual are histamine-2 antagonists and regular insulin. Fe dextran is also sometimes added to dextrose/amino acid mixtures simply is incompatible with TNA. In add-on, pharmacists are often queried regarding Y-site compatibility of diverse drugs with PN solutions. The reader is referred to a standard reference text for data regarding compatibility of drugs with PN solutions.12
Determination
PN, a potentially lifesaving therapy, is sometimes combined with intake via the oral or tube route. Some physicians nevertheless utilize PN in situations where no SNS is required, such as in previously adequately nourished patients who are expected to resume oral intake within a week. Other physicians underuse EN and instead prescribe PN in patients with a functional gut. In patients requiring PN, the pharmacist will be chosen upon for expertise, especially when stability and compatibility issues ascend. While the corporeality of dextrose and lipid supplied in PN has decreased over the years, the value of supplying substantial protein is still recognized. Since parenteral micronutrient requirements are sometimes difficult to determine, PN requires careful monitoring. The emerging importance of tight glycemic command in hospitalized patients is some other challenge for clinicians managing PN.
REFERENCES
ane. Dudrick SJ. A 45-year obsession and passionate pursuit of optimal nutrition support: puppies, pediatrics, surgery, geriatrics, home TPN, A.Southward.P.E.N., et cetera. J Parenter Enteral Nutr. 2005;29:272-287.
2. A.S.P.E.N. Lath of Directors. Guidelines for the use of parenteral and enteral diet in adult and pediatric patients. J Parenter Enteral Nutr. 2002;26(one Suppl) 1SA-138SA.
3. Grant JP. Parenteral admission. In: Rombeau JL, Rolandelli RH, eds. Clinical Nutrition: Parenteral Nutrition. 3rd ed. Philadelphia: WB Saunders Company; 2001:109-117.
4. Orr ME. The peripherally inserted cardinal catheter: what are the current indications for its apply? Nutr Clin Pract. 2002;17:99-104.
5. Culebras JM, Garcia-de-Lorenzo A, Zarazaga A, et al. Peripheral parenteral diet. In: Rombeau JL, Rolandelli RH, eds. Clinical Nutrition: Parenteral Nutrition . 3rd ed. Philadelphia: WB Saunders Company; 2001:580-587.
6. Driscoll DF, Adolph M, Bistrian BR. Lipid emulsions in parenteral nutrition. In: Rombeau JL, Rolandelli RH, eds. Parenteral Nutrition. 3rd ed. Philadelphia: WB Saunders Company; 2001:35-59.
7. Holcombe BJ, Gervasio JM. Developed parenteral nutrition. In: Koda-Kimble MA, Young LY, Kradjan WA, et al., eds. Practical Therapeutics: The Clinical Use of Drugs. eighth ed. Philadelphia: Lippincott Williams & Wilkins; 2005;37-1–37-23.
8. Mirtallo JM. Parenteral formulas. In: Rombeau JL, Rolandelli RH, eds. Parenteral Diet. 3rd ed. Philadelphia: WB Saunders Company; 2001:118-139.
9. Task strength for the revision of safe practices for parenteral nutrition. Prophylactic practices for parenteral nutrition. J Parenter Enteral Nutr. 2004;28:S39-S70.
ten. Parenteral multivitamin products. Federal Register. April 20, 2000;65:21200-21201.
11. Klein GL. Aluminum contamination of parenteral nutrition solutions and its impact on the pediatric patient. Nutr Clin Pract. 2003;18:302-307.
12. Trissel LA. Handbook on Injectable Drugs. 13th ed. Bethesda, MD: American Society of Health-Arrangement Pharmacists; 2005.
thirteen. O'Grady NP, Alexander One thousand, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. MMWR. 2002;51(RR-10):1-26.
xiv. Sacks GS, Driscoll DF. Does lipid hang time make a difference? Time is of the essence. Nutr Clin Pract. 2002;17:284-290.
15. Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? J Parenter Enteral Nutr. 1987;eleven:viii-13.
sixteen. Dickerson RN. Specialized nutrition back up in the hospitalized obese patient. Nutr Clin Pract. 2004;19:245-254.
17. Krenitsky J. Adjusted trunk weight, pro: evidence to back up the use of adjusted trunk weight in computing calorie requirements. Nutr Clin Pract. 2005;twenty:468-473.
18. Wooley JA, Btaiche IF, Good KL. Metabolic and nutritional aspects of acute renal failure in critically sick patients requiring continuous renal replacement therapy. Nutr Clin Pract. 2005;20:176-191.
19. Timoney JP, Malkin MG, Leone DM, et al. Rubber and cost effective apply of alteplase for the clearance of occluded central venous access devices. J Clin Oncol. 2002;20:1918-1922.
20. Couban Due south, Goodyear G, Burnell Yard, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of primal venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;20:4063-4069.
21. Klerk CP, Smorenburg SM, Buller HR. Thrombosis prophylaxis in patient populations with a central venous catheter: a systematic review. Curvation Intern Med. 2003;163:1913-1921.
22. Anderson AD, Palmer D, MacFie J. Peripheral parenteral nutrition. Br J Surg. 2003;90:1048-1054.
23. Kraft MD, Btaiche IF, Sacks GS, Kudsk KA. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Wellness Syst Pharm. 2005;62:1663-1682.
24. Fessler TA. Trace element monitoring and therapy for adult patients receiving long-term total parenteral nutrition. Pract Gastroenterol. 2005;44:51-52,54,56,58,60,63-65.
25. van den Berghe M, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically sick patients. North Engl J Med. 2001;345:1359-1367.
26. McMahon MM. Management of parenteral nutrition in acutely sick patients with hyperglycemia. Nutr Clin Pract. 2004;19:120-128.
27. Talpers SS, Romberger DJ, Bunce SB, Pingleton SK. Nutritionally associated increased carbon dioxide production. Excess full calories vs high proportion of carbohydrate calories. Chest. 1992;102:551-555.
28. Buchman A. Total parenteral diet-associated liver affliction. J Parenter Enteral Nutr. 2002;26(v Suppl):S43-S48.
29. Seidner DL. Parenteral diet-associated metabolic os disease. J Parenter Enteral Nutr. 2002;26:S37-S42.
30. Mermel LA, Farr BM, Sherertz RJ, et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249-1272.
To comment on this commodity, contact editor@uspharmacist.com.
Source: https://www.uspharmacist.com/article/parenteral-nutrition
0 Response to "what is the highest amount of dextrose to administer through a picc line."
Post a Comment